https://abc11.com/nc-coronavirus-reopen-cases-update/6132539/
Same story…T should have known about upcoming (now everywhere Pandemic) with early warning from US Intelligence.
U.S. intelligence agencies issued warnings about the novel coronavirus in more than a dozen classified briefings prepared for President Trump in January and February, months during which he continued to play down the threat, according to current and former U.S. officials.
The repeated warnings were conveyed in issues of the President’s Daily Brief, a sensitive report that is produced before dawn each day and designed to call the president’s attention to the most significant global developments and security threats.
For weeks, the PDB — as the report is known — traced the virus’s spread around the globe, made clear that China was suppressing information about the contagion’s transmissibility and lethal toll, and raised the prospect of dire political and economic consequences.
But the alarms appear to have failed to register with the president, who routinely skips reading the PDB and has at times shown little patience even for the oral summary he now takes two or three times per week, according to the officials who spoke on the condition of anonymity to discuss classified material.
PR parade today at T presser…
When asked about T’s comment about disinfectant…
AND THIS from OANN…T’s mouthpiece
And more dumping on China
And the net net of today’s presser
FDA pushed through scores of inaccurate antibody tests without agency review
Some are giving too many false positive results, which could mislead some people into thinking they have already been infected.
The CDC revised its symptoms list nearly two weeks ago. No announcement was made, and so this is only becoming known NOW. This list would have allowed thousands more to potentially be tested.
CDC confirms six coronavirus symptoms showing up in patients over and over
Mass. General Hospital released a coronavirus simulator. Here’s what it shows for Massachusetts.
Researchers predict a late-summer spike if restrictions are loosened too soon.
Beyond sad…
“She tried to do her job, and it killed her,” said the father of Dr. Lorna M. Breen, who worked at a Manhattan hospital hit hard by the coronavirus outbreak.
The elder Dr. Breen said his daughter had contracted the coronavirus but had gone back to work after recuperating for about a week and a half. The hospital sent her home again, before her family intervened to bring her to Charlottesville, he said.
…
“She was truly in the trenches of the front line,” he said.
He added: “Make sure she’s praised as a hero, because she was. She’s a casualty just as much as anyone else who has died.”
In a statement, NewYork-Presbyterian/Columbia used that language to describe her. “Dr. Breen is a hero who brought the highest ideals of medicine to the challenging front lines of the emergency department,” the statement said. “Our focus today is to provide support to her family, friends and colleagues as they cope with this news during what is already an extraordinarily difficult time.”
A New York hospital struggling against the coronavirus says PPE price gouging is so bad that it’s paying $7 for gowns worth 50 cents, and $25 for shields worth $1.25
Nurses protest for protective equipment at White House

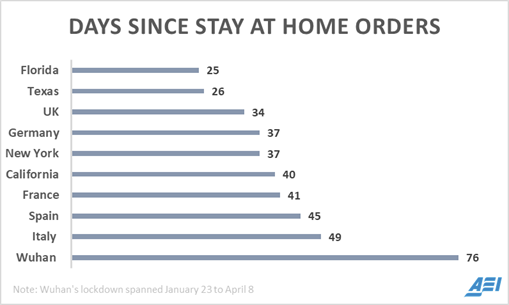
76 days
McDonald Trump won’t use the Defense Production Act to make vital PPE and other products our hospitals need, but he’ll use it to force meat plants that closed because of rampant Covid-19 outbreaks from closing.
Priorities, right?
Trump to order meat plants to stay open in pandemic, person familiar with action says
Facilities will be declared ‘critical infrastructure’ for their role in the nation’s food supply even as many become virus hot spots
Governor DeWine (R-OH) is listening to the scientists and not on the T-loyalty wagon…which is a big departure for such a R leaning state. One-by-one they peel away from T’s insanely reasoned handling of this pandemic.
Now, Mr. DeWine is charting a way out of the shutdown, taking cautious steps while facing pressure from business leaders, conservative activists and some Republican lawmakers who vociferously question the economic costs of a state in quarantine.
Seven weeks into the crisis, Mr. DeWine is being guided by health experts while avoiding partisan fissures over stay-at-home orders that have been encouraged by Mr. Trump, who hopes a rebounding economy will carry him to re-election. The Ohio governor is the rare Republican official who does not automatically fall in step with Mr. Trump, an independence he shares with two other Republican governors, Larry Hogan of Maryland and Charlie Baker of Massachusetts, both of whom lead solidly Democratic states where bipartisanship is needed to survive. Unlike them, Mr. DeWine has gone his own way in a red-hued state.
He also split decidedly with Mr. Trump by encouraging a nearly all-mail primary election on Tuesday. While the president has spread the false claim that voting by mail entails “a lot” of fraud, Mr. DeWine pushed universal absentee ballots for voters’ safety. Ohio’s secretary of state on Monday called the effort a success, with nearly 1.5 million mail ballots cast.
So after early findings showed Gilead’s remdesevir held promise, the WHO released a report contradicting this, and now a report is out confirming the first position, and Dr. Fauci has backed it.
I don’t know what to think. I rather hope it really is a breakthrough, though.
Fauci Calls Early Data From Gilead Virus-Drug Trial ‘Good News’



So many of are most vulnerable are affected by this.
1.
2.
3.
4.
Meanwhile… this:
5.
Glad that the House Oversight Committee is going after the FDA on their lax standards for allowing so many anti-body tests to be released without thorough testing of these. I am hearing that serum testing has done better than finger pricking tests…but again, efficacy (the ability to produce an intended outcome) needs to be reviewed. They are asking for a review back by May 6th.
A House subcommittee is driving up pressure Wednesday on companies marketing coronavirus antibody tests that may fail to meet “a reasonable standard of accuracy.”
Rep. Raja Krishnamoorthi (D-Ill.), chairman of the House Oversight subcommittee on economic and consumer policy, wrote to four companies — including one that’s issuing tests in partnership with a major American medical tech company — requesting details of the firms’ contact with the Food and Drug Administration, data supporting the accuracy of their tests and a list of medical facilities who have purchased test kits from the companies.
>
The outreach is part of Krishnamoorthi’s investigation into the FDA’s handling of the antibody tests, which are meant to help researchers determine the extent of the coronavirus outbreak by finding people who have been exposed and might have developed immunity. It’s a crucial datapoint for researchers as they seek to guide the U.S. coronavirus response and determine when it is safe to begin relaxing stringent social distancing and stay-at-home orders around the country.“The Subcommittee is concerned that FDA is not conducting substantive review of serological tests that it has allowed on the market and that those tests may not meet a reasonable standard of accuracy,” Krishnamoorthi said in the letters, which were sent to North Carolina-based BioMedomics, San Diego’s Epitope Diagnostics, Minnesota-based Premier Biotech and San Jose’s UCP Biosciences.
The FDA waived its typical quality checks to help antibody tests reach the market quickly, instead allowing companies to self-evaluate the accuracy of their products. Researchers have begun issuing evidence that many of the tests are falling short of typical accuracy standards, producing false positives that could lead people to believe they’ve developed protection against the virus.
Krishnamoorthi pointed to evidence obtained by University of California researchers that tests marketed by the companies he’s targeting fall short of the accuracy they claim in their marketing materials and instructions for use. In addition, UCP Biosciences has not been identified by the FDA as one of the 150 tests allowed on the market, yet its tests were purchased by a 25-bed hospital in rural Kansas, according to local reports.
An earlier staff report by the panel raised alarms about the lack of controls on the market for antibody tests, warning of “fraudulent” products and scolding the FDA for issuing “unclear guidance” while failing to police the market for these tests. And in a new letter to FDA Commissioner Stephen Hahn, Krishnamoorthi demands by May 6 information about the FDA’s process for retroactively reviewing antibody tests that have come to market, whether any have met the agency’s standards for approval and whether any have been removed or faced enforcement actions because they fell short.
And I think we should “protect” the president first & foremost…
Absolutely! I’ll have to type them out and get back to you🥰
